- Categories:Company news
- Author:
- Origin:
- Time of issue:2019-07-17
- Views:0
(Summary description)On July 11, 2019, Ningbo Aocheng Biotechnology Co., Ltd.'s new detection product-Placental Growth Factor Detection Kit (Immunofluorescence) (hereinafter referred to as "Aucheer iRaTe PlGF") was officially approved for listing. The product was recommended by the International Federation of Obstetrics and Gynecology (FIGO) on June 18, 2019, which issued the strongest level recommendation of the practical guidelines for early pregnancy screening and prevention, opening a new chapter in the scientific and standardized management of hypertension and preeclampsia during pregnancy in my country
(Summary description)On July 11, 2019, Ningbo Aocheng Biotechnology Co., Ltd.'s new detection product-Placental Growth Factor Detection Kit (Immunofluorescence) (hereinafter referred to as "Aucheer iRaTe PlGF") was officially approved for listing. The product was recommended by the International Federation of Obstetrics and Gynecology (FIGO) on June 18, 2019, which issued the strongest level recommendation of the practical guidelines for early pregnancy screening and prevention, opening a new chapter in the scientific and standardized management of hypertension and preeclampsia during pregnancy in my country
- Categories:Company news
- Author:
- Origin:
- Time of issue:2019-07-17
- Views:0
On July 11, 2019, Ningbo Aocheng Biotechnology Co., Ltd.'s new testing product —— Placental growth factor detection kit (immunofluorescence method) (Hereinafter referred to as "Aucheer iRaTe PlGF") was officially approved for listing. The product was recommended by the International Federation of Obstetrics and Gynecology (FIGO) on June 18, 2019, which issued the strongest level recommendation of the practical guidelines for early pregnancy screening and prevention, opening a new chapter in the scientific and standardized management of hypertension and preeclampsia during pregnancy in my country.

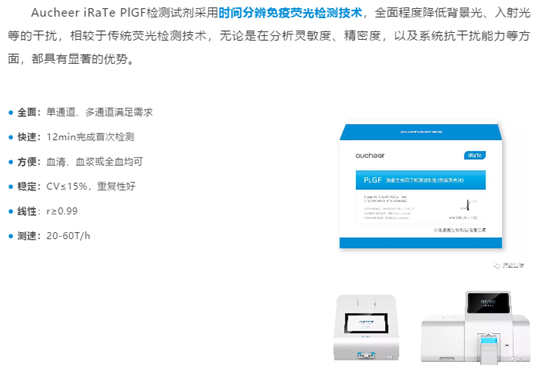
Aucheer iRaTe PlGF detection reagent used time-resolved immunofluorescence detection technology,It can reduce the interference of background light and incident light to a comprehensive extent. Compared with traditional fluorescence detection technology, it has significant advantages in terms of analysis sensitivity, precision, and system anti-interference ability.
Early screening and prevention throughout pregnancy to ensure the safety of mothers and babies
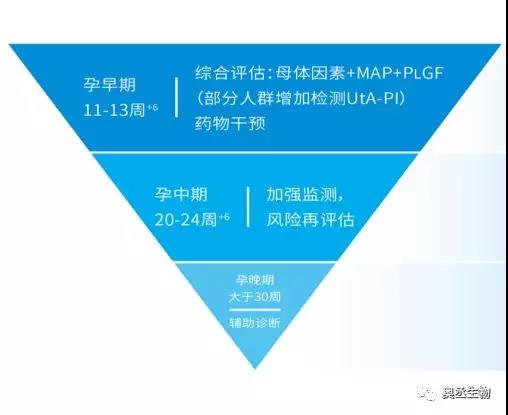
The Aucheer iRaTe PlGF test combines maternal factors to perform early pregnancy risk screening and predictive monitoring in the second and third trimesters, so as to assist clinicians in more reasonable risk stratification and clinical assessment of high-risk factors and pregnant women with suspected preeclampsia, and to achieve high-risk pregnant women. More scientific prevention and management can reduce or delay the occurrence and development of preeclampsia and ensure the safety of mothers and fetuses.

Create a new era in the prevention and treatment of preeclampsia in China
In the near future, Aocheng will establish in-depth cooperation with medical institutions and academic experts at all levels across the country to develop a series of faster and more accurate preeclampsia risk assessment and management testing products, and explore a preeclampsia risk assessment for pregnant women in China With the road of management and prevention, the incidence of hypertension and preeclampsia during pregnancy in my country can be effectively controlled and greatly reduced.